A Deep Learning Approach to Early Alzheimer’s Forecasting in the Wild
Introduction
Alzhiemer’s Disease (AD) is a brain degenerative disease which causes 60-70% of cases of dementia [@pmid2397368] and affects an estimated 6.7 million people age 65 and older [@pmid36918389]. Drug and non-drug treatments have been conducted symptomatically against dementia in the late stage of AD [@pmid36918389] [@VAZ2020173554], but they are inefficacious to modify the progression of AD [@VAZ2020173554]. On the other hand, disease-modifying therapies (DMT) focusing on interventions based on the amyloid cascade hypothesis and tau biology [@pmid30501965] are able to preserve cognitive and functional capacity of the patients, but they require accurate diagnosis at the pre-dementia and preclinical stages [@pmid31994640], the identification of which is still a challenging topic in the neuroscience community [@pmid23183885]. Since recent interdisciplinary studies demonstrate successful application of machine learning analysis to medical data including images [@pmid28301734] and time series [@pmid33693379], we propose a deep learning approach to analysis subjects’ longitudinal records and determine the subjects’ risk in developing AD over time. The risk factors generated by our model then suggest whether subjects will develop AD in the future, serving as indicators of clinical trail recruitment and DMT early application.
Significance
Clinical medicine
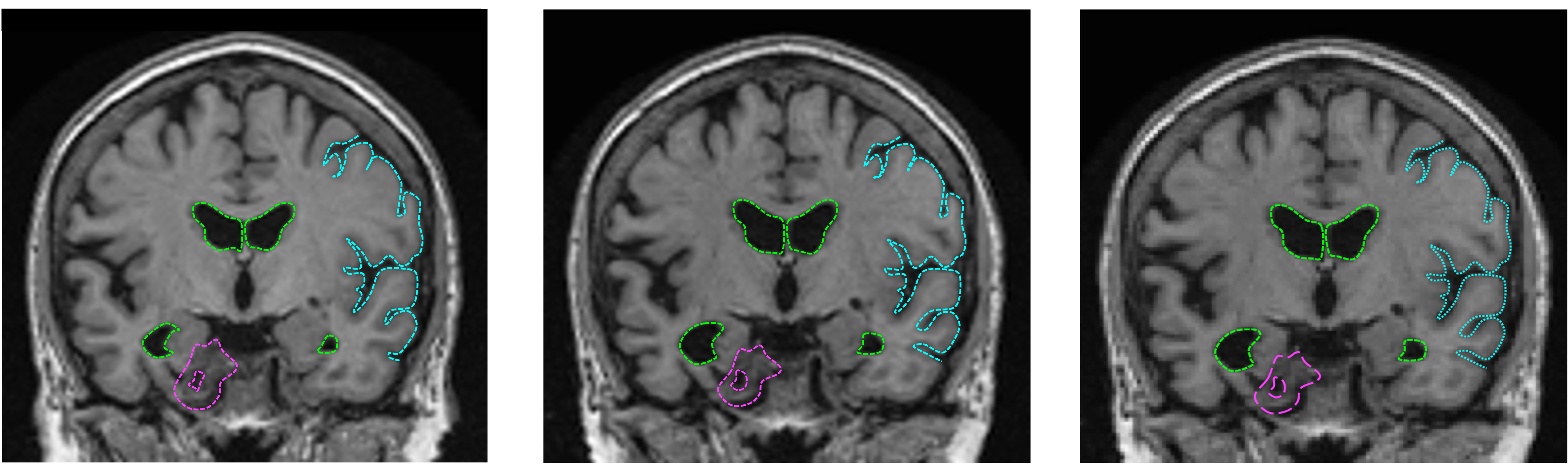
At present, no method – mathematical, clinical, or epidemiological – predicts the development of AD, limiting the identification of cohorts to conduct early symptomatic treatments and DMT. Since the majority of AD treatments happens in the late stage [@VAZ2020173554][@pmid35286590], the damage caused by the disease to the brain is generally irreversible. For example, without early AD diagnosis and early intervention, amyloid-$\beta$ cascade often has already produced cognitive symptoms before treatments are applied, resulting in limited success in pharmacotherapies targeting amyloid-$\beta$ and tau in the late stage of the disease [@pmid33268824]. Also, degenerative pathology cased by late-onset AD is observed on hippocampal volume and cortical thickness in several brain regions computed from MRIs [@pmid30217936] (figure [fig:mridegen]{reference-type=”ref” reference=”fig:mridegen”}). To assist early AD diagnosis, our deep learning model will be able to predict future AD stages from the observed subjects’ medical records and allow 1) the identification of patient cohorts for potential recruitment of clinical trials; and 2) the testing of early interventions and medications that interrupt the course of AD.
Neuroscience
There are keen efforts in neuroscience research on the early detection of AD. Some of the well studied key risks include mild cognitive impairment (MCI) subtype (e.g., aMCI, naMCI), poor performance on various neurocognitive tests, and biomarkers such as abnormal cerebrospinal fluid (CSF) tau or tau/amyloid-$\beta$ ratio, APOE4 positive status, white matter hyperintensities, and atrophy in the hippocampal, medial temporal, or entorhinal regions [@pmid35286590] [@pmid30284855] [@pmid30320579] [@pmid31682146]. To analyse numerous risk factors efficiently, data-driven AD progression models were proposed and revealed multifactorial interactions between various risk factors and disease progressions [@pmid30321505]. We take a further step towards this interdisciplinary approach by utilizing the high capacity of deep learning models to 1) process high volumes of data of multiple modalities including cognitive test scores, demograpchis, biomarkers, and volumetric features computed from magnetic resonance imaging (MRI) on regions of interest; 2) introduce full MRI modality besides MRI based engineered features via computer vision and information theory techniques; and 3) capture future health states transitions and mapping from health states abstraction to a diagnosis as an assistance to neuroscience research.
Related Deep Learning Work
Recently, applying deep learning models to AD studies has been addressing increasing interests. From a computer vision perspective, 2D [@Valliani] [@JAIN2019147] or 3D [@liu2020design] convolutional neural networks (CNN) are applied to MRI 2D slices or full MRIs to extract latent representations for AD classification. People also study AD future progress detection and forecasting via time series models such as recurrent neural networks (RNN) [@ALBRIGHT2019483] [@flare], bidirectional-RNN [@RAHIM2023363] and long short-term memory networks (LSTM) [@ABUHMED2021106688], reaching a maximal forecasting window of 2.5 years.
Potential Translational Impact
The proposed deep learning model that forecasts future AD stages can be naturally applied to multiple real-world scenarios. a) By integrating the model to electronic health record management systems, the model can perform AD stages forecasting per patient utilizing the historical records stored in the system in a regular time interval, say 6 months, and will be able to raise early alerts once future dementia is detected. b) The model can be embedded into MRI machines and do real-time analysis once MRIs are captured. c) When pharma companies or neuroscience researchers identify individuals to be recruited for clinical trails, our model can serve as an assistance software that takes available individual’s records and outputs the estimated future AD progression, so that clinical trails can recruit more patients with light or no cognitive issues but progressing pathological changes. In summary, upon completion, this project will make it possible to inform patients of their risks in a timely manner and select subjects for clinical trials.